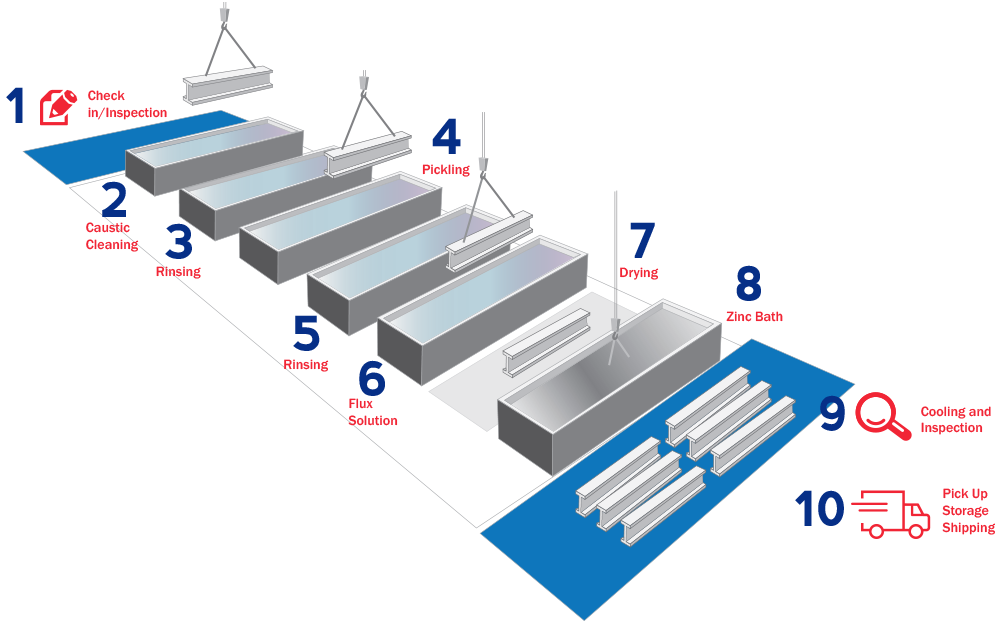
Galvanizing Process
Applying the hot dip Galvanizing process to steelwork provides fabrications with a robust, durable and corrosion protective finish that under normal conditions will last for many years without maintenance of any sort. The process itself has a number of stages that are required to achieve the final finish.
These are all by immersion and they may be summarized as follows :

DEGREASING
It uses either acid or alkaline-based proprietary products and heat or used at ambient temperatures. The target is to produce a surface, which is not contaminated with Oil, or Grease based products.
PICKLING
The process of Picking is carried out in dilute hydrochloric acid which dissolves rust and scale and produces a ‘chemically clean’ surface which will react with the molten zinc.


FLUXING
A mixture of zinc chloride and ammonium chloride in solution is the standard fluxing agent of choice. Fluxing is the final surface preparation step in the galvanizing pretreatment process. Fluxing prevents further oxides from forming on the surface of the metal prior to galvanizing.
Drying Oven
This process also heats the steel to between 60-80 degrees Celsius which helps the steelwork to dry after it is withdrawn. Drying is important as it helps prevent zinc splash and a separate drying stage is sometimes employed


ZINC IMMERSION
This ‘final’ stage utilizes a special bath holding molten zinc at 450°C. The clean steel is immersed in the zinc and while it is submerged it alloys with the iron in the steel to form zinc/iron alloy layers. These layers form the basis of the coating, which is then overlain with free zinc, as it is withdrawn from the galvanizing bath. The coating that is formed offers cathodic protection to the steel to which it is alloyed. As such, it will corrode preferentially to the steel extending its life by many years.

